Epitope mapping is the process of experimentally identifying the binding site, or “epitope”, of an antibody on its target antigen (usually, on a protein). Epitope characterization data can also help elucidate the mechanism of action for an antibody and can strengthen intellectual property (patent) protection. NovaBioAssays provides state-of-art instrumentation and service for HDX-MS (hydrogen-deuterium exchange mass spectrometry) technology. HDX-MS is suitable for determining both linear and conformational epitopes and is the most efficient way to determine conformational epitopes for large and complicated (e.g. heavily glycosylated) antigens in native buffer condition. Sometimes, the antibodies not only bind to the target but trigger agonistic/antagonistic effects by changing the antigen’s conformation. HDX-MS is also capable of elucidating such conformation changes.
To date, we have successfully identified epitopes for over 60 antigens with hundreds of antibodies, including popular targets covering multiple disease areas such as PD1, HER2, PCSK9 and SARS-COV2-RBD. The monomeric molecular weight of the antigens was ranging from 6kDa to 190 kDa, with various degrees of glycosylation, PTMs and tags for expression. The general turnaround time of one antigen with one antibody is 2-3 weeks. We also support customized HDX-MS instrumentation requests and provide our protease columns as catalogue items.
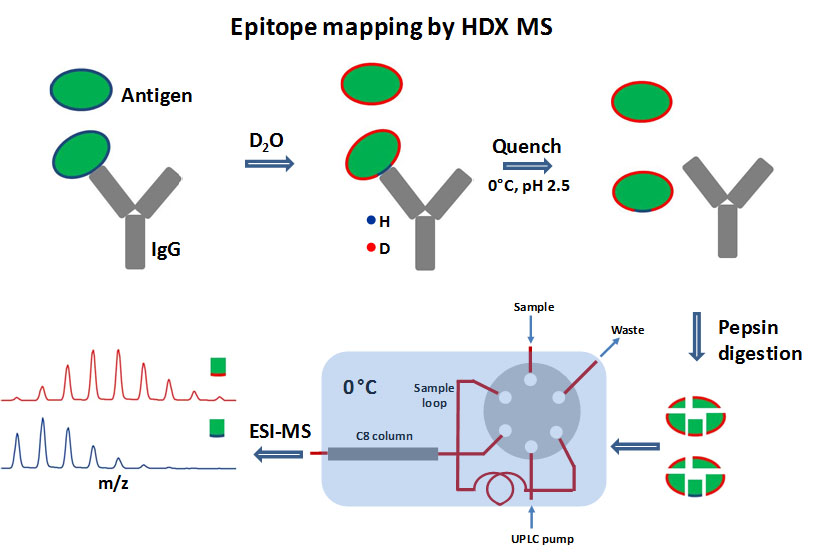
Briefly, the workflow involves automated steps for deuterium labeling, quenching, online enzymatic digestion, and LC-MS. In particular, the online enzymatic digestion step was performed by our own variety of low-pH active protease columns, allowing excellent sequence coverage for difficult proteins. The liquid chromatography step was performed at precisely -9 °C or lower temperature to enable minimized back-exchange level with a relative long 30-minute UPLC gradient. The high-resolution Q Exactive Plus mass spectrometry and advanced bioinformatics tools can then be used to detect and analyze most of the peptides, including the ones with glycosylation modifications.
