Non-regulated Bioanalysis
Early discovery and preclinical bioanalysis are critical for making decisions in lead selection/optimization during the drug discovery cycle. Bioanalysis is comprised of quantitative methods that measure drug and metabolite concentrations for samples in different biological matrices, including plasma, serum, cerebrospinal fluid (CSF), urine, and various tissue.
For non-regulated bioanalysis, we help the clients perform method development, method transfer (from and to clients), method qualification and sample analysis. We provide services with both traditional ELISA based approach and mass spectrometry-based protein bioanalysis. We are the industry leader of mass spectrometry-based protein bioanalysis and perform various in-house developed unique assays to tackle difficult projects such as quantifying low levels of analytes in multiple tissue with or without immunoprecipitation reagents. We are also capable of quantifying multiple analytes in the same sample using multiplexed workflow such as measuring the antibody and payload distribution in tissue for ADC. Some of our previously delivered assays are described in the table here. We collaborate with the clients to provide fit-to-purpose solutions to meet the requirements for assay performance and timeline.
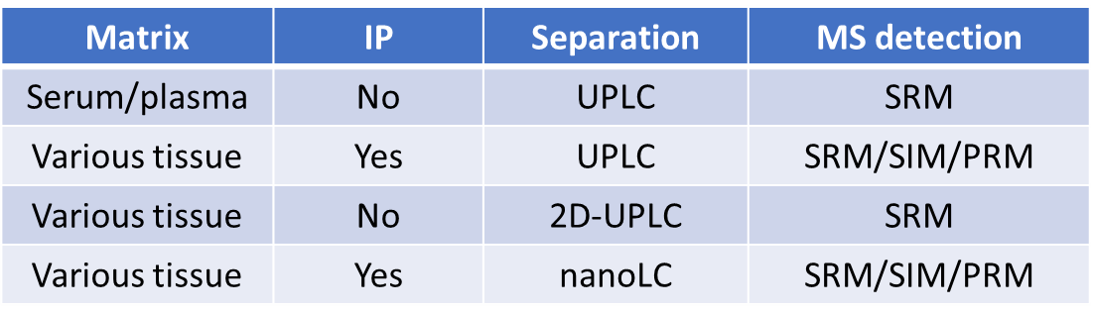 IP: Immunoprecipitation
IP: Immunoprecipitation
UPLC: Ultra performance liquid chromatography
SRM: Single reaction monitoring by triple quadruple mass spectrometry
SIM: Selected ion monitoring by orbitrap mass spectrometry
PRM: Parallel reaction monitoring by orbitrap mass spectrometry