NovaBioAssays provides the LC-MS or LC-MS/MS based analytical characterization services for Antibody-Drug Conjugates (ADCs) and other therapeutic proteins, such as bispecific antibodies, Fc fusion proteins, antibody fragments (Fab, Fc, and Nanobodies), etc. We provide a suite of biophysical, chromatography and mass spectrometry methods to characterize aggregation, charge variants, purity, sequence, clipping/PTM and glycosylation for the analyte protein. Selected services are:
-
SEC-UV, SEC-MALS, and native SEC-MS
-
IEX-UV, native IEX-MS, and iCIEF-UV for charge variant
-
HIC-UV for hydrophobic variant
-
Intact Molecular Weight Determination
-
Peptide Mapping
-
Post Translational Modification(PTM) Analysis
-
Disulfide Bridge Mapping
-
Glycosylation Analysis and Glycosylation Site Mapping/Occupancy Determination
-
Protein Identification
In addition to the assays characterizing regular therapeutic proteins, we provide following services for ADC:
-
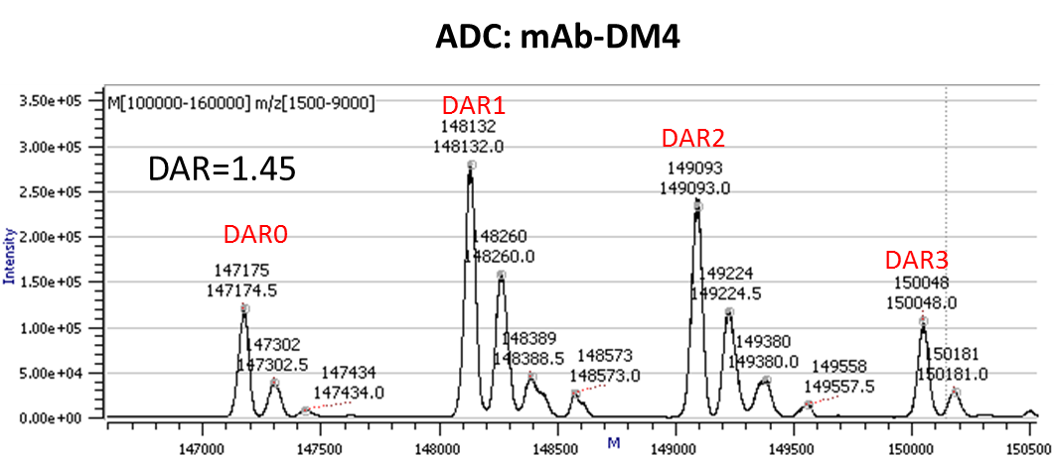
Drug-to-Antibody Ratio (DAR) Determination (example shown in figure below)
-
Site Occupancy
-
Linkage site identification
-
Analysis of Free Drug (Payload) and Naked Carrier Protein/Antibody
-
Quantification of Drug-Conjugate Components